Study on the Hygroscopicity of Polyacrylamide and Its Porous Materials
1 Introduction
Organic polymer hygroscopic materials are new types of functional polymer materials that were originally developed from high water-absorbing resins. It has excellent hygroscopicity and moisture retention properties and is an aqueous resin modified by chemical and physical methods to absorb water by hydrophilic groups in the molecule. The ability of organic macromolecular hygroscopic materials to absorb moisture from the air is higher than that of ordinary inorganic hygroscopic materials, the moisture absorption capacity can reach over 70%, the moisture absorption rate can reach 2mg/g·min, and there is no corrosion, pollution, and regeneration [1~3] . In addition, the variety of product forms (powdery, granules, strips, or transparent films) of organic polymeric hygroscopic materials can be used in different applications. Meet different moisture needs.
Polyacrylamide has the same number of side groups as the number of acrylamide units in the molecular chain - the acylamino group. Amido groups readily form hydrogen bonds with water or substances containing -OH groups (natural fibers, proteins, soil, and minerals), resulting in strong adsorption. Due to its strong hygroscopicity, PAM is used as a class of organic polymeric hygroscopic materials. At present, hygroscopic materials at home and abroad have weak points such as poor hygroscopicity and failure to recycle, and no one systematically studies organic macromolecular hygroscopic materials. This article systematically studied the effect of various polymerization conditions on the moisture absorption properties of PAM and obtained the best process for polymerization. This will have guiding significance for the development of hygroscopic materials with fast moisture absorption, high moisture absorption capacity and no corrosion.
2 Test Methods and Test Plans
2.1 Raw materials
Acrylamide (AM), AR, Beijing Yili Fine Chemicals Co., Ltd.; Sodium hydroxide, AR, Beijing Beihua Fine Chemicals Co., Ltd.; Sodium sulfite: AR, Beijing Yili Fine Chemicals Co., Ltd.; Ammonium persulfate, AR , Beijing Chemical Plant; N, N'-methylenebisacrylamide (MBAA), AR, Beijing Hongxing Venus Chemical Factory; color-changing silicone (A-type), Qingdao Ocean Chemical Co., Ltd.; molecular sieve (3A), Dalian Haixin Chemical Co., Ltd.; polyethylene glycol PEG200, AR, Shanghai Pudong Gaonan Chemical Plant; calcium carbonate (CaCO3), AR, Beijing Hongxing Chemical Factory; deionized water.
2.2 Preparation of Polymers
2.2.1 Preparation of PAM
First, according to the formula, put the weighed monomer (AM) into the beaker, stir it well, add the cross-linking agent MBAA and the initiator [Na2SO4-(NH4)2S2O8] in turn, stir it well, and adjust it with a certain concentration of NaOH solution. pH to neutral. Weigh the total weight, make up the expected amount with deionized water, and put it into the reaction tube. The nitrogen is discharged in warm water and oxygen is discharged, and then it is placed in a constant temperature water bath to react.
2.2.2 PAM/PEG Preparation
Put the metered monomer AM into the beaker, stir well, add the crosslinker MBAA and initiator [Na2SO4-(NH4)2S2O8] in turn, stir well, then adjust the pH to neutral with a certain concentration of NaOH solution, and finally Add porogen polyethylene glycol. Weigh the total weight and fill it up with deionized water. In warm water, nitrogen was exhausted for 30 minutes. The solution was poured into a homemade container and reacted in an oven at 60°C for 4 hours.
2.2.3 Preparation of PAM/CaCO3
Put the metered monomer AM into the beaker, stir well, add the crosslinker MBAA and initiator [Na2SO4-(NH4)2S2O8] in turn, stir well, then adjust the pH to neutral with a certain concentration of NaOH solution, and finally Add porogen CaCO3. Weigh the total weight and make up for the estimated amount with deionized water. In warm water, nitrogen was exhausted for 30 minutes. The solution was poured into a homemade container and reacted in an oven at 60°C for 4 hours. The sample was soaked in a 0.1 mol/L hydrochloric acid solution and the hydrochloric acid solution was changed every 3 days until CaCO3 was completely washed out. Distilled water is then used to remove excess acid to give the final product.
The resulting reaction gel was washed, sectioned, granulated, dried (T=120°C), crushed and sieved to make a sample.
2.3 Hygroscopic Property Test
The 10 g sample oven was weighed to the balance and weighed accurately to 0.001 (operating time ≤ 5 s, operating environment humidity ≤ 50 RH). After placing the sample in a constant temperature and humidity chamber, it begins to absorb moisture. Weigh once every 1 h until the end of the test when the two successive weighings do not differ by more than 5 mg. Under the specified relative humidity conditions, the moisture absorption rate of the moisture-absorbing material is calculated by the following formula: 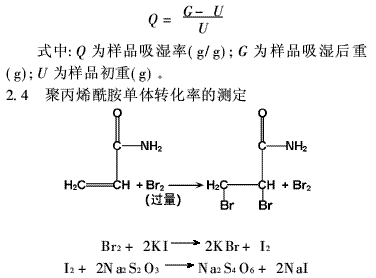
2.4.1 Configuration of Bromine
Weigh 10g KBr, 2.79g KBrO3, dissolved in a 1000ml volumetric flask, dilute to the mark.
2.4.2 Measurement Procedure
Accurately weigh 0.05g PAM sample into a 250ml ground-mouth conical flask and add appropriate amount of distilled water. Accurately add 20 ml bromine solution from the burette. Then add 4~5ml 3mol/L sulphuric acid solution, shake well and place it in dark place for 2~3h. Then add 10ml of 20% potassium iodide solution, shake, titrate with 0.05mol/L sodium thiosulfate standard solution until the solution changes from brownish red to light yellow, add 0.2ml 0.5% starch solution, continue to titrate until the blue disappears. To the end. At the same time as a blank experiment. Monomer Conversion (mass %) X is calculated as follows: 
According to the above method according to 0, the conversion rate of the PAM monomer was determined to be over 75%.
3 Results and Discussion
3.1 Hygroscopic principle
As shown in Figure 1, the moisture absorption rate of PAM is faster than that of molecular sieves and silica gel, and the moisture absorption curves of silica gel and molecular sieves are close to the level after 150 minutes. This indicates that silica gel and molecular sieve have reached the maximum moisture absorption capacity but the values ​​are small. This is because silica gel and molecular sieves are non-elastic gels, have a porous capillary structure, a large specific surface area, and exhibit a strong adsorption capacity. The main reason for their adsorption is that the atomic force field on a solid surface is not saturated and has surface energy. Therefore, water molecules can be adsorbed to reduce the surface energy.
Silica gel contains Si-O bonds. Once Si-O bonds come into contact with moist air, the silicon atoms on the surface react with water to produce hydroxyl groups, which are chemically adsorbed water; in addition, there is also physically adsorbed water on the surface, but Since silica gel is a non-elastic gel, it does not swell after moisture absorption, and its capacity for moisture absorption is limited, which restricts its ability to absorb moisture. Silica gel and molecular sieves can only absorb moisture by physical adsorption. This process is performed only on the surface and cannot penetrate into the interior. Therefore, the moisture absorption capacity is greatly reduced. 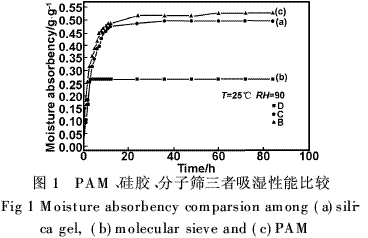
The moisture absorption process of PAM includes physical adsorption and chemical adsorption. The physical adsorption and adsorption rate is fast, and the chemical adsorption rate is slow. Because of its shape network structure, the surface area is relatively small compared to silica gel and molecular sieves, so the initial moisture absorption rate is not fast. However, as the moisture absorption process continues, chemical adsorption occurs after the physical adsorption ends, and the moisture adsorbed on the PAM surface slowly permeates through the osmotic pressure, so that the PAM's moisture absorption performance is greatly improved.
According to the different types of binding of water molecules and functional groups, the “three-state water model [4]†that is widely recognized is the combination of water, bound water, and free water, as shown in Fig. 2. The absorption of water on the surface of the hydrophilic group is approximately 0. to 0.6 nm. The first layer is the binding water in which the polar group forms a coordination bond or hydrogen bond with the water molecule, and the second layer is a binding water layer in which the water molecules align and align under the action of the polar force field. The outermost layer is free. water. Because the PAM has a higher water absorption capacity, it is far beyond the physical absorbents such as silica gel and molecular sieves, so its maximum moisture absorption capacity will also be very large. V is water molecules; A is not frozen water; B is bound water; C is free water 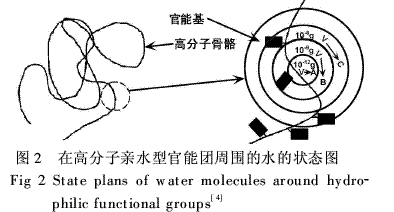
3.2 Effect of reaction conditions on moisture absorption
3.2.1 Effect of Bulk Concentration on Moisture Absorption Performance
Polymers prepared at different monomer concentrations have very different hygroscopic properties (Figure 3). The monomer concentration was between 20% and 26%. With the increase of monomer concentration, the moisture absorption rate increased significantly and reached the maximum value at 26%. When the monomer concentration was >26%, it decreased. This is because the change in the monomer concentration affects the degree of crosslinking and the molecular weight of the polymerization product. Usually, the concentration is low, the cross-linking reaction is not easy to proceed, the molecular weight of the product is also low, and the product is partially soluble in water, resulting in a low moisture absorption rate. When the monomer concentration is too high, the molecular weight of the polymerization reaction decreases, and the degree of crosslinking increases, so that the moisture absorption rate decreases. 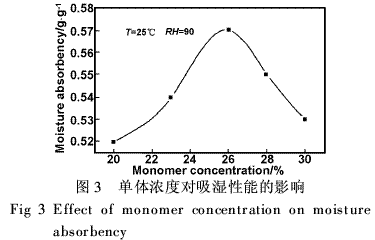
3.2.2 Effect of Crosslinking Agent on Moisture Absorption Properties
Fig. 4 is a graph obtained by changing the amount of the cross-linking agent without changing the monomer composition ratio. When the amount of cross-linking is 0.12%, the moisture absorption rate of PAM is the highest. When the cross-linking agent is ≤0.12%, the moisture absorption rate will increase significantly with the increase in the amount of cross-linking agent; when the amount of cross-linking agent is ≥0.12%, the opposite is true. According to PJ Flory's formula, the increase of cross-linking agent dosage increases cross-linking density and increases Ve/V0, which results in three-dimensional space structure, smaller pore size of the macromolecular network, and shorter cross-linking length, thus lowering the moisture absorption rate. On the other hand, if the amount of cross-linking agent is reduced, the pore size of the macromolecular network becomes large, the cross-linking is prolonged, and the relative moisture absorption rate is high. 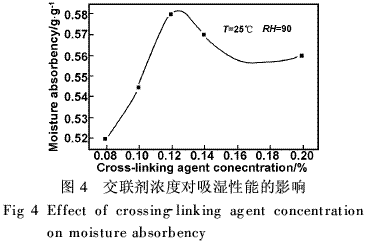
3.2.3 Effect of Urea Content on Moisture Absorption Figure 5 shows the effect of urea content on the hygroscopicity of PAM. The moisture absorption rate of PAM decreased first with urea content and then decreased, reaching the highest value at a content of about 30%. The addition of urea prevents the formation of cross-links between polymer molecules, resulting in insolubles and insolubles, and is more conducive to the formation of an effective three-dimensional network structure. Therefore, as the urea content increases, the moisture absorption rate also increases. However, excessive addition of urea results in a decrease in the molecular weight, which results in a decrease in effective moisture-absorbing groups, and therefore a decrease in the moisture absorption rate. 
3.2.4 Effect of Degree of Hydrolysis on Moisture Absorption Properties
Figure 6 shows the effect of the degree of hydrolysis on the moisture absorption rate of PAM. The difference in the degree of hydrolysis leads to a difference in the pH of the solution. The pH of the solution has a great influence on the hygroscopicity of the PAM. At different pH conditions, the polymerization and the properties of the final product are quite different. 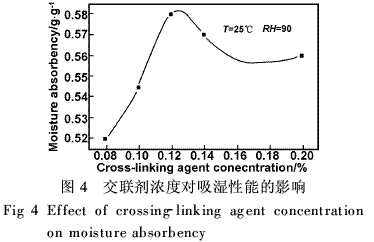
From Table 1, the increase in the degree of hydrolysis leads to an increase in the pH of the solution. The moisture absorption rate of PAM increases as the pH of the solution increases. A significant feature of the alkaline hydrolysis of PAM is that the orthogroup effect of the anionic carboxyl group causes the hydrolysis reaction to exhibit a self-blocking effect, which is in contrast to the positive catalysis of the PAN's acidic hydrolysis of the ortho, and the hydrolysis rate is significantly slowed as the degree of hydrolysis increases. This is because the electrostatic repulsion of the nucleophilic group OH-, which is an anion-type carboxylate group introduced on the main chain, reduces the effective concentration of OH- in the local microenvironment around the acylamino group [5].
This ortho-base effect causes alkaline hydrolysis of PAM, and the hydrolysis rate is very fast when the degree of hydrolysis is <20%. In an alkaline environment, the activation energy of growth reaction EP increases with increasing pH [6]. When the pH of the polymerization solution is high, the amide bond of the monomer AM is prone to hydrolysis and is negatively charged. The repulsive effect between the static charges changes the conformation and the ability of the active site of the growing radical, and the corresponding chain growth and growth reaction activates. Can increase the EP. Therefore, the moisture absorption rate of PAM increases as the degree of hydrolysis increases. 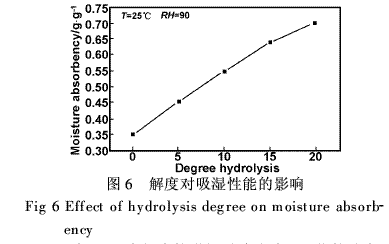
3.2.5 Effect of Polymerization Temperature on Moisture Absorption Performance
As shown in Figure 7, the optimum reaction temperature is about 50°C. A too high or too low polymerization temperature has a great influence on the properties of the product. According to the reaction kinetics, the relationship between the rate constant of the chemical reaction and the reaction temperature is as follows: 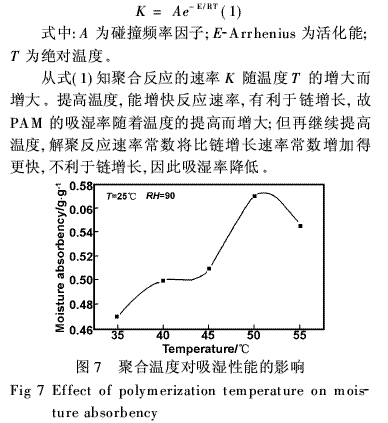
3.2.6 Effect of Porogen on the Moisture Absorption of PAM
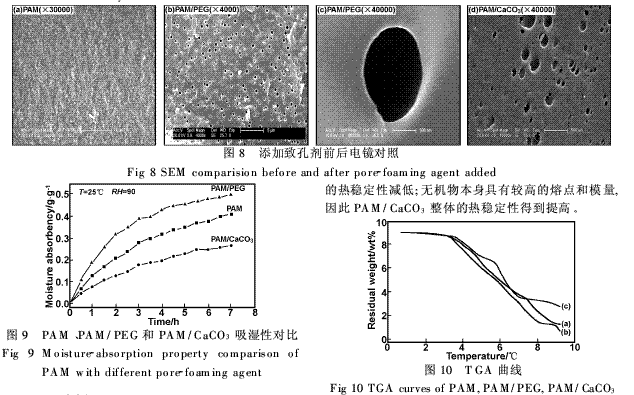
The classification of pores was first proposed by Dubinin [7] and later by IUPAC: pore width <2 nm is micropores; between 2 and 50 nm mesopores;> 50 nm macropores. The PAM without porogen has no pores on the surface (Fig. 8(a)). Hygroscopicity mainly depends on the interaction of the hydrophilic group with water and the osmotic pressure difference between the inside and outside of the network to absorb water, so the moisture absorption rate is not high.
The addition of PEG pore-forming agent resulted in a uniform distribution of macropores on the surface of the PAM (Figure 8(b)), and the effective moisture-absorbing surface area was greatly increased; whereas, the Ca-CO3 porogen produced only sparsely-distributed mesopores. 8(c)). As shown in Figure 9, the moisture absorption rate of PAM/PEG is more than 50% faster than that of PAM, and the moisture absorption capacity is also significantly increased. However, the moisture absorption effect of PAM/CaCO3 is not ideal. The most important parameter that determines the amount of adsorption is the surface area, and the presence and abundance of mesopores directly affect the specific surface value, because the width of the mesopores is much larger than the general molecule. The interaction between the adsorbent surface and the adsorbent molecules in the mesoporous adsorbent is limited to a distance not far from the surface [8], and monolayer or multi-layer adsorption occurs.
4 TGA Analysis
TGA curves for PAM, PAM/PEG, and PAM/CaCO3 are shown in Figure 10. After adding PEG, the thermal stability of PAM/PEG was slightly lower than that of PAM, while the thermal stability of PAM/CaCO3 was slightly improved. Because PEG200 itself has a relatively low melting point, PAM/PEG
5 Conclusion
The specific surface area is one of the important parameters affecting the moisture absorption performance. With the addition of PEG porogen, uniform pores appear on the surface of the PAM/PEG material, so the moisture absorption performance is greatly improved. However, the cavitation effect of CaCO3 is not obvious, so the moisture absorption effect of PAM/CaCO3 is not ideal. The test results at T = 20°C and RH = 90% show that the moisture absorption performance of PAM is significantly better than that of traditional silica gel and molecular sieves, both in terms of moisture absorption capacity and moisture absorption rate, thus demonstrating the superiority of organic macromolecular hygroscopic materials. TGA analysis showed that the thermal stability of PAM/PEG was slightly lower than that of PAM, while the thermal stability of PAM/CaCO3 was slightly increased.
SpO2 Moduel,Temerature Moduel,Monitor Moduel
Shanghai Berry Electronic Tech Co., Ltd , http://www.chequipments.com
